A medical device is supposed to be a tool for hope, a way to recover, or a chance for a new normal. When one fails, it can cause a physical and emotional setback that leaves you wondering where to turn next.
 The companies that make these devices have a duty to ensure they are safe, effective, and honestly labeled. When they fail in that duty, a defective medical device creates a painful spiral of new injuries, surgeries, and stress you never expected, and aren’t prepared to handle.
The companies that make these devices have a duty to ensure they are safe, effective, and honestly labeled. When they fail in that duty, a defective medical device creates a painful spiral of new injuries, surgeries, and stress you never expected, and aren’t prepared to handle.
At Freese & Goss, we step in when patients feel overwhelmed and unheard. With more than 77 years of combined experience and over $1 billion recovered for clients, our team knows how to take on major medical device manufacturers, no matter how big they are.
If a medical device has caused you new pain or problems, you deserve more than a generic answer or a rushed phone call. Our Dallas defective medical device lawyers take the time to really hear your story and help you feel in control again. Reach out to us today online or by calling (214) 761-6623.
“Excellent service and attention. Professional, clear, and committed. They helped me resolve my case successfully. 100% recommended.”
– Sergio C. | Client
Why These Cases Need the Right Dallas Defective Medical Device Attorney on Your Side
Defective medical device cases aren’t simple—they’re some of the most technical and hard-fought cases in personal injury law. You’re not just dealing with an injury. You’re going up against a major medical device company with deep pockets, large legal teams, and a lot to lose.
Here’s what sets these cases apart, and why working with an experienced firm like Freese & Goss can give you the support you need:
1. These Cases Are Highly Technical
It’s not enough to show that you were hurt; you have to show how the device failed. That means your legal team must understand medical records, surgical procedures, and engineering reports. We work with respected medical experts and product engineers to prove how a device was defective and how exactly that defect caused your injuries.
These cases also require looking into a manufacturer’s history and decisions, including:
- Clinical Trials: Reviewing whether the device was properly tested before being sold.
- Internal Emails and Documents: Looking for evidence that the company knew about the dangers but chose to hide them or ignore them to rush the product to market.
- FDA Submissions: Analyzing the path the company took to get the device approved, often through the less rigorous 510(k) pathway, which does not require new testing if the device is “substantially equivalent” to an older one.
2. Facing Down Powerful Corporations
Medical device manufacturers are massive, multi-billion-dollar companies. They have extensive legal teams whose only goal is to minimize their liability and make your case go away as cheaply as possible.
When you hire a firm with a national reputation like Freese & Goss, you send a clear message: you are ready to fight. Our firm has the resources to stand toe-to-toe with these giants, covering the costs required for expert testimony, depositions, and litigation that can take months or even years.
3. Understanding Mass Torts and MDLs
Many defective medical device cases involve a mass tort, meaning a large number of people across the country have been injured by the same device. These cases are often grouped together in a Multi-District Litigation (MDL) in federal court. High-profile mass torts we have experience in include Zantac, Roundup, and Dupixent.
Unlike a class action, where everyone shares the same settlement, an MDL allows each person’s case to be handled individually. Our attorneys have been appointed to multiple Plaintiffs’ Steering and Executive Committees for major multi-district litigations, giving us unique insights into how these complex cases are properly managed and won.
Examples of Defective Medical Devices
A medical device can be almost anything used in medicine, from a simple surgical tool to an implanted mechanism. A defect can occur in any of them, leading to lasting patient harm.
Over the years, many devices have been involved in lawsuits, including:
- Joint Replacements (Hip and Knee Implants): Certain metal-on-metal hip implants, for example, were found to shed toxic metal debris into a patient’s bloodstream, a condition called metallosis. This can cause severe tissue damage, bone loss, and the need for painful and complex revision surgery.
- Surgical Mesh (Hernia and Transvaginal Mesh): These flexible net-like implants, meant to support weakened tissue, have caused painful complications like mesh erosion, contraction, infection, and damage to surrounding organs, often requiring multiple removal surgeries.
- IVC Filters: These tiny spider-like devices are inserted into the body’s main vein (the vena cava) to prevent blood clots from reaching the lungs. If defective, they can fracture, tilt, migrate, or even pierce the vein wall, leading to life-threatening complications.
- Pacemakers and Defibrillators: Devices designed to keep the heart beating properly can fail due to faulty batteries, wiring issues, or programming errors, causing electrical malfunction and potential death.
- Insulin Pumps and Glucose Monitors: Defects in these devices can lead to inaccurate drug delivery, causing dangerous high or low blood sugar levels, resulting in life-threatening conditions for diabetic patients.
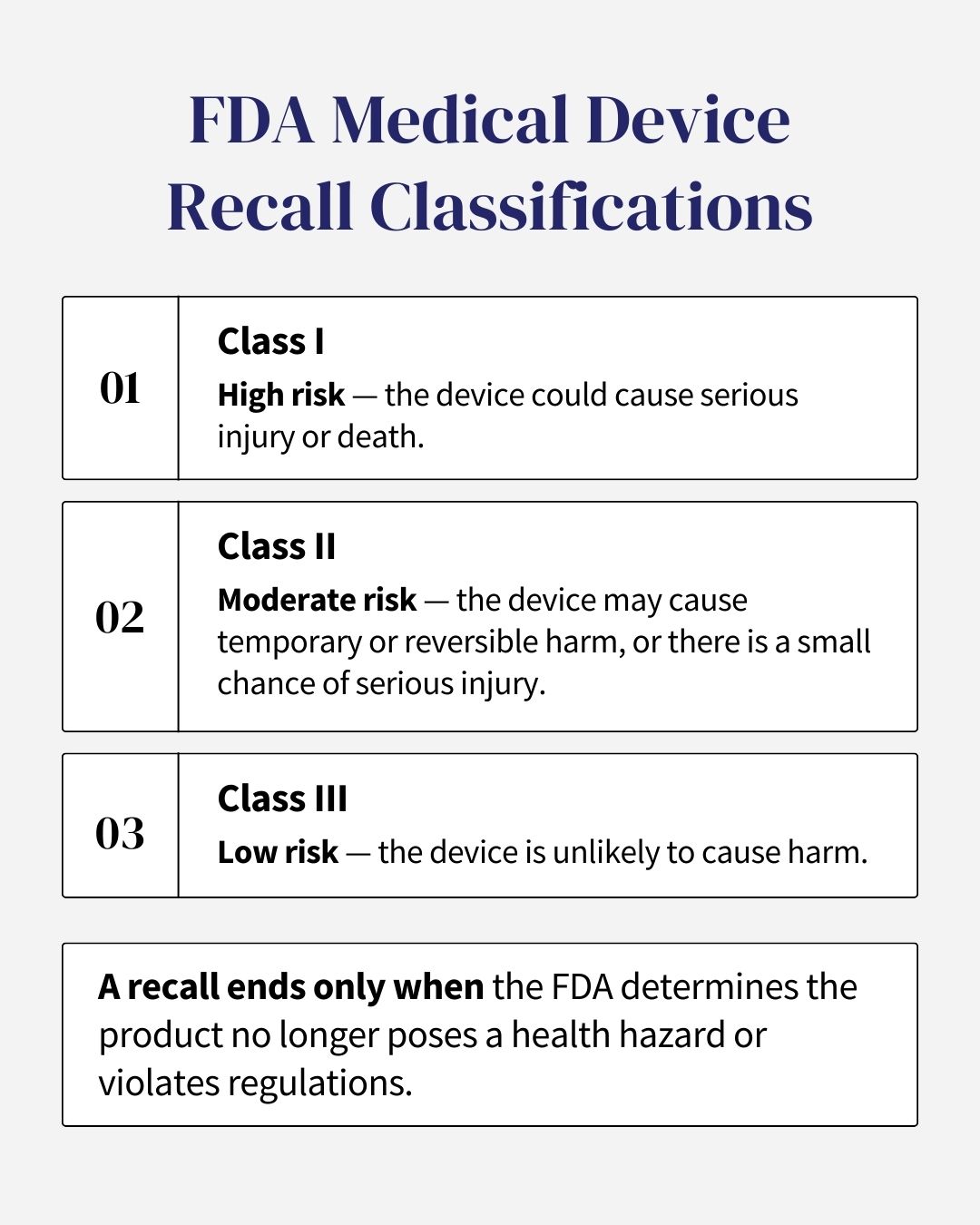 What Are the Reasons Behind a Medical Device Recall?
What Are the Reasons Behind a Medical Device Recall?
Recalls are triggered by defects that fall into one of three main categories:
- Manufacturing Defect: This means there was an error during the actual making or production of the device. For example, a batch of pacemakers was made with a faulty battery, or a hip implant was contaminated during assembly.
- Design Defect: This is a problem with the blueprint or plan of the product, meaning that even if the device is built perfectly, the design itself is inherently unsafe. For example, a type of surgical mesh was designed with a material that causes a toxic reaction in the human body.
- Marketing Defect (Failure to Warn): The product is safe on its own, but the manufacturer failed to provide proper warnings or instructions about the known dangers or risks. For example, a company knows its device is not safe for certain patient groups but fails to clearly state this in the packaging or to doctors.
The FDA receives over two million medical device reports of suspected deaths, serious injuries, and malfunctions each year. The fact that a recall happened is often powerful evidence of a defect, though it does not automatically guarantee a successful lawsuit.
What to Do If My Medical Device Was Recalled?
If you are a patient in Dallas and receive a notice that your implanted or used medical device has been recalled, you need to act immediately, but with caution:
- Call Your Doctor: The first and most important step is to contact the doctor or surgeon who implanted the device. They can advise you on the specific risks and whether or not the device needs to be monitored, adjusted, or surgically removed. Do not make any changes to the device or your medication without a doctor’s guidance.
- Keep All Documentation: Save the recall notice, all communications from the manufacturer, any paperwork from your doctor, and all medical bills.
- Do Not Throw the Device Away: If the device is surgically removed, ask the hospital to preserve it as evidence.
- Contact a Dallas Defective Medical Device Attorney Right Away: A recall can significantly strengthen your legal claim. Call Freese & Goss to discuss whether you may qualify for a defective medical device claim.
In 2024, medical device recalls hit a four-year peak, with 1,059 incidents reported, according to Sedgwick’s 2025 U.S. State of the Nation Recall Index.
Who Can a Dallas Defective Medical Devices Lawyer Hold Responsible?
Several different companies may have played a part in putting a dangerous device in your hands, and our job is to find out exactly who.
The potentially responsible parties our Dallas defective medical device lawyers will investigate include:
- The Manufacturer: This is almost always the main target. They design, test (or fail to test), and produce the device.
- Component Part Suppliers: Sometimes a defect isn’t in the main device, but in a specific part, like a faulty battery or a contaminated piece of metal provided by a different company.
- The Distributor or Wholesaler: These companies move the product from the manufacturer to the hospital or store. If they knew about a defect and failed to act, they could be held responsible.
- The Hospital or Retailer: In some cases, the facility or store that sold the product could be liable, especially if they failed to handle the device correctly or ignored warnings.
A doctor is usually not the main focus of a defective device case. But if a surgeon used the wrong size device, placed it incorrectly, or failed to warn you about risks they knew about, they may share responsibility. Most of the time, though, these cases come back to the company that designed or made the device.
Figuring out who’s truly at fault isn’t always simple, and you shouldn’t have to guess. If you’re dealing with a device that failed you, our team can sort through the details and explain your options in plain language. Call (214) 761-6623 or reach out through our online contact form to get answers you can trust.
Compensation a Dallas Defective Medical Device Lawyer May Help You Recover
Many people go into surgery hoping for relief, only to end up facing revision surgery, more pain, and the crushing feeling of taking steps backward instead of forward.
If a defective medical device has turned your recovery into a painful uphill battle, you have the right to seek compensation for every way this has changed your life.
A Dallas defective medical device lawyer at Freese & Goss will work to pursue the full value of your losses, which may include:
- Past and Future Medical Expenses: All costs tied to your injury, including the first surgery, any revision or removal surgery, hospital stays, doctors’ visits, physical therapy, medications, and future care you’ll need down the road.
- Lost Wages and Future Income: Income you’ve missed while recovering, plus any future loss of earning ability if your injuries limit the kind of work you can do.
- Pain and Suffering: The physical pain, ongoing discomfort, and emotional toll caused by the defective device and the procedures required to fix it.
- Emotional Distress: Anxiety, depression, trauma, sleep problems, and the distress caused by the defective medical device.
- Loss of Enjoyment of Life: Compensation for the activities, hobbies, or everyday moments you can no longer enjoy because of your injuries.
- Punitive Damages: In rare cases where a manufacturer acted with extreme recklessness or ignored known risks, a jury may award punitive damages to hold the company fully accountable.
Why So Many Injured Patients Turn to Freese & Goss
At Freese & Goss, we never lose sight of our real priorities: protect you, fight for you, and make sure powerful companies don’t get away with hurting the people they promised to help.
Of course, we’re proud of our results, but what matters most is what those results mean for real people who were struggling, scared, and unsure of what to do next.
We’ve taken on national manufacturers in some of the toughest defective device cases, and we’ve delivered life-changing outcomes for clients. For example:
- $33,684,140 Verdict: We helped secure the first-ever plaintiff’s verdict against Rex Medical for its defective IVC filter.
- $5,700,000 Verdict: This was the first trial win in the country involving Ethicon’s TVT-Abbrevo pelvic mesh device.
With over 77 years of combined experience, Freese & Goss lead a team that focuses solely on serious injury and defective device cases. We don’t dabble. This is our work and our passion.
Don’t wait and risk losing your rights. Call (214) 761-6623 for a free, confidential consultation, or reach out through our online contact form. We’re here to be your steady, experienced team when everything else feels uncertain.

